About the projects
- Home
- About the projects
The Partnership for Excellence in Cancer Research between the University of Puerto Rico (UPR) and the University of Texas MD Anderson Cancer Center (MDACC) builds on the long-standing relationship and shared vision of the two institutions to eradicate cancer health disparities and promote health equity for underserved communities and socio-economically disadvantaged populations in Puerto Rico, Texas, and elsewhere. To address some of the most profound cancer health disparities in these communities, the Partnership established the Infection-Driven Malignancies Program for Advancing Careers and Translational Sciences (IMPACT) by leveraging on the breadth and strengths of research expertise in both institutions.
Hispanics have an increased risk of infection-driven malignancies, including gastric cancer, and HIV- and HPV-related malignancies. To address the increased risk factors and burden of infection-driven cancers that exist among Hispanic populations in the US, the Partnership strives to reduce these disparities through efforts in Cancer Research, Education, and Outreach. Our research projects employ novel approaches, include joint multidisciplinary and transdisciplinary research teams with faculty members from both institutions, and aim at enhancing research capacity in Puerto Rico. Our projects are:
- Full Project 1
- Full Project 2
- Pilot Project
- Pilot Project 2
- Pilot Project 3
- Pilot Project 4
- Full Project 3
Title: Understanding and Targeting of Convergent Immunosuppressive Pathways and Molecular Signaling in HPV-positive and HPV-negative Penile Cancer
Project Leaders: Magaly Martinez-Ferrer, PhD (UPR) and Curtis Pettaway, MD (MDACC)
Penile cancer is a highly morbid disease that exhibits a higher mortality among Puerto Ricans than among the rest of the US population. Although infection with human papillomavirus (HPV) has been identified as a risk factor for penile cancer, the etiology of penile cancer is incompletely understood. Therefore, we recently developed the first genetically engineered mouse (GEM) models of penile squamous cell carcinoma (PSCC), the predominant histologic type of penile cancer, through co-deletion of tumor suppressor genes (SMAD4 and APC) in mouse penile epithelium. We have also generated the first set of patient-derived xenograft (PDX) models for penile cancer (N=6). In our pilot project, using this GEM model of PSCC, we found: (1) substantial infiltration of immune cells in the penile tumors, especially myeloid-derived suppressor cells (MDSCs) that can inhibit cytotoxic T cells and cause immune evasion, and (2) strong cyclooxygenase-2 (COX2) expression in penile tumors and PI3K/mTOR signaling in MDSCs. In addition, using expression arrays we identified novel insights into expression patterns associated with human HPV-positive and HPV-negative penile cancer. The objective of this proposal is to develop therapeutic strategies for penile cancer and validate molecular pathways associated with HPV-positive and HPV-negative penile cancer subtypes. Our hypotheses are: (1) Penile cancer formation is promoted by chronic inflammation as a result of HPV infection or downregulation of essential tumor suppressor genes (SMAD4 and APC); (2) The key signaling hubs driving penile cancer progression, including COX2 and PD-L1 upregulation, are effective targets for immunotherapeutic intervention; and (3) Estrogen and Notch signaling are upregulated in HPV-positive penile cancer and play important roles in penile cancer progression. The specific aims of this proposal are to: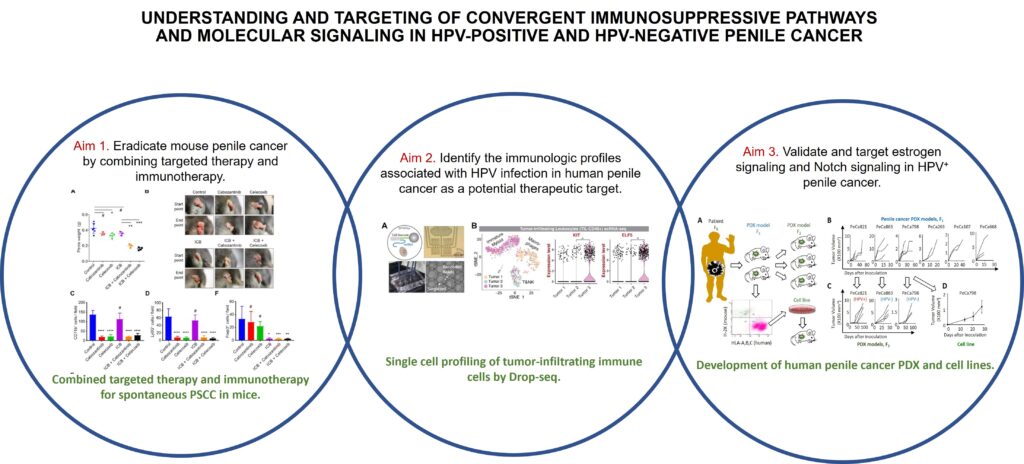
The proposed studies will have a significant impact on both the understanding of the molecular pathways that drive HPV-positive and HPV-negative cancer subtypes and the identification of effective therapeutic strategies to treat these highly morbid tumors. Through this unique collaboration our multidisciplinary research teams from The University of Texas MD Anderson Cancer Center and the University of Puerto Rico are poised make novel contributions to understanding and curing this rare fatal cancer.
Selected publications related to this project:
- Chahoud J, Gleber-Netto FO, McCormick BZ, Rao P, Lu X, Guo M, Morgan MB, Chu RA, Martinez-Ferrer M, Eterovic AK, Pickering CR, Pettaway CA. 2021. Whole Exome Sequencing in Penile Squamous Cell Carcinoma Uncovers Novel Prognostic Categorization and Drug Targets Similar to Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. Jan 13: clincanres.4004.2020. doi: 10.1158/1078-0432.CCR-20-4004. PMID: 33441293.
- Huang T, Cheng X, Chahoud J, Sarhan A, Tamboli P, Rao P, Guo M, Manyam G, Zhang L, Xiang Y, Han L, Shang X, Deng P, Luo Y, Lu X, Feng S, Martinez-Ferrer M, Wang YA, DePinho RA, Pettaway CA, Lu X. 2020. Effective Combinatorial Immunotherapy for Penile Squamous Cell Carcinoma. Nature Communications.11, 2124. https://doi.org/10.1038/s41467-020-15980-9
- Colón-López, V, Ortiz, AP, Soto-Salgado, M, Torres-Cintron M, Pettaway CA, Puras-Baez A, Martínez-Ferrer M, Suárez, E 2012. Penile cancer disparities in Puerto Rican men as compared to the United States population. International Brazilian Journal of Urology, 38(6): 728-38. PMID: 23302411, PMCID: PMC3703478
- Svatek RS, Munsell M, Kincaid JM, Hegarty P, Slaton JW, Busby JE, Gaston KE, Spiess PE, Pagliaro LC, Tamboli P, Pettaway CA. Association between lymph node density and disease specific survival in patients with penile cancer. J Urol 2009. 182(6):2721-7. PMID: 19837433.
- Bermejo C, Busby JE, Spiess PE, Heller L, Pagliaro LC, Pettaway CA. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol 2007. 177(4)(4):1335-38. PMID: 17382727
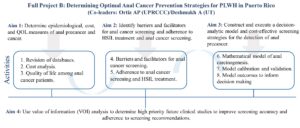
Title: Determining Optimal Anal Cancer Prevention Strategies for HIV-Infected Individuals Living in Puerto Rico
Project leaders: Ana P. Ortiz, PhD (UPR) and Scott B. Cantor, PhD (MDACC)
Squamous cell carcinoma of the anus (SCCA) is an epidemic among persons living with HIV (PLWH). Studies in the US and Puerto Rico have reported >80-fold risk of SCCA among PLWH when compared with the general population. Guidelines based on expert opinion recommend anal cancer screening for PLWH. To inform evidence-based use of screening, studies are currently underway; however, findings from those studies will not be generalizable to PLWH in Puerto Rico. This is because racial and ethnic composition, disease prevalence, anal cancer treatment outcomes including cost and quality of life (QOL) substantially differ for PLWH in Puerto Rico. This increases the importance of anal cancer screening evaluation specifically and separately for PLWH in Puerto Rico, the majority of which are of Hispanic origin. Furthermore, no ongoing or future randomized controlled trials will evaluate mortality benefits of screening, as it unethical to evaluate a “no screening” arm. As such, a mathematical model (incorporating epidemiological, cost, and quality of life data specific to PLWH in Puerto Rico could be utilized as a unique tool to evaluate survival and QOL benefits and cost-effectiveness of screening with the goal to promote health and value-based care. Our overarching goal is to optimize anal cancer prevention for PLWH in Puerto Rico. We will study the epidemiology of SCCA, and evaluate the cost of and QOL after SCCA treatment to inform a mathematical model that will determine the best screening regimen for PLWH in Puerto Rico. We also seek to study critical barriers and facilitators necessary to decrease potential uncertainty in screening adoption and adherence to screening recommendations.
In Aims 1 and 2, we will perform secondary data analyses of existing databases, and conduct focus groups and three epidemiologic studies (two cross-sectional studies and one cohort study) that involve patient recruitment. Parameters identified through these activities will inform the development of the SCCA screening model and the execution of the VOI analysis (Aims 3 and 4). The end result of this study will be utilized to inform an optimal anal cancer screening regimen specific to PLWH in PR.
Selected publications related to this project:
- Colón-López V, Shiels MS, Machin M, Ortiz AP, Strickler H, Castle PE, Pfeiffer RM, Engels EA. Anal Cancer Risk Among People With HIV Infection in the United States. J Clin Oncol 2018. 36(1);68-75. PMID: 29140774, PMCID: PMC5791846.
- Ortiz AP, Engels EA, Nogueras-González, GM, Colón-López V, Soto-Salgado M, Vargas A, Machin M, Shiels MS. Disparities in HPV-related cancer incidence and survival among HIV-infected Hispanics living in the United States. Cancer 2018. 124(23);4520-4528. PMID: 30345506
- Ortiz AP, Pérez-Irizarry J, Soto-Salgado M, Suárez E, Pérez N, Cruz M, Palefsky J, Tortolero-Luna G, Miranda S, Colón-López V. Human papillomavirus-related cancers among people living with AIDS in Puerto Rico. Prev Chronic Dis 2014. 11:E80. PMID: 24831284, PMCID: PMC4023685.
- Ortiz AP, Ortiz-Ortiz KJ, Traverso-Ortiz M, Ríos MY, Colón-López V, Palefsky JM. Anal cancer trends in Puerto Rico from 1985 to 2005: the potential impact of the AIDS epidemic. AIDS Patient Care STDS 2014. 28(4):165-7. PMID: 24660788, PMCID: PMC3985527.
- Deshmukh AA, Chiao EY, Cantor SB, Stier EA, Goldstone SE, Nyitray AG, Wilkin T, Wang X, Chhatwal J. Management of precancerous anal intraepithelial lesions in human immunodeficiency virus-positive men who have sex with men: Clinical effectiveness and cost-effectiveness. Cancer 2017. 123(23):4709-4719. PMID: 28950043 PMCID: PMC5693634.
- Deshmukh AA, Cantor SB, Fenwick E, Chiao EY, Nyitray AG, Stier EA, Goldstone SE, Wilkin T, Chhatwal J. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: The time is now. Vaccine 2017. 35(38):5102-5109. PMID: 28807605 PMCID: PMC5581672.
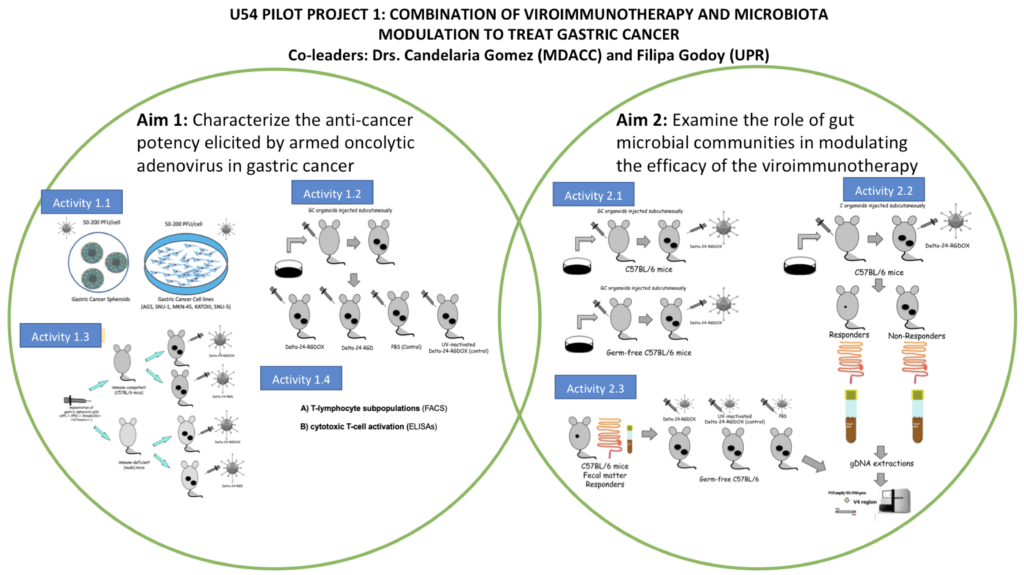
Title: Combination of Viroimmunotherapy and Microbiota Modulation to Treat Gastric Cancer
Project leaders: Filipa Godoy-Vitorino, PhD (UPR) and Candelaria Gomez-Manzano, MD (MDACC)
Gastric cancer is the third leading cause of cancer-related mortality, with a 5-year survival rate of approximately 20%. The incidence and mortality rates of gastric cancer in the U.S. are of high concern, especially among non-white populations. Hispanic, black non-Hispanic, and Asian/Pacific Islander populations have a 40-50% higher risk of gastric cancer than White people, and African Americans are nearly twice as likely to die of gastric cancer. Virotherapy, as a special case of immunotherapy, is showing promising results for solid tumors in clinical trials. We developed an oncolytic adenovirus, Delta-24-RGD, which was clinically tested in a first-in-human phase I clinical trial in patients with recurrent glioblastoma. Clinical trials and preclinical
Studies showed that the intratumoral injection of Delta-24-RGD triggered an anti-tumor immune response that induced complete tumor regression in a small but significant percentage of patients. These clinical data emphasize the need to develop strategies that will significantly increase the percentage of solid tumors like gastric cancer sensitive to virotherapy. Recent studies showed that the intestinal microbiota influence the efficacy of immunotherapy. These clinical data have been supported by rigorously controlled experiments using gnotobiotic mouse models colonized with one or more specific bacteria, which showed that certain microbial biomarkers were associated with modulating and enhancing anti-tumor therapies, such as improving efficacy of immunotherapy. These data suggest that therapeutic interventions aimed at altering the gut microbiome may influence the final clinical outcome. Here, we hypothesize that oncolytic adenoviruses will exert an effective anti-cancer effect in gastric cancer, and that the host gut microbiome plays an important role in modulating the virus-driven, anti-tumor response. To test this hypothesis, we propose the following aims:
- Aim 1. Characterize the anticancer-potency elicited by armed oncolytic adenovirus in gastric cancer.
- Aim 2. Examine the role of gut microbial communities in modulating the efficacy of the viroimmunotherapy.
In Aim 1, we will utilize the Delta-24-RGD platform of replication-competent, tumor-selective adenoviruses, and the next generation of Delta-24-RGD armed with the immunomodulator OX40L, Delta-24-RGDOX. In Aim 2, we will assess the anticancer effect of the oncolytic therapy in relation to different bacterial signatures. The data generated by this project should yield new information about the potential use of oncolytic adenoviruses as therapy for gastric cancer and will open avenues to include intestinal microbiota as a potential treatment modifier, by maximizing the synergy between laboratories at the University of Puerto Rico (UPR) and M.D. Anderson Cancer Center (MDACC). Our pilot project is aligned with the Infection-Driven Malignancies Program for Advancing Careers and Translational Sciences (IMPACT), in that it will allow us to generate preliminary data with potential to be translated into a full project to address a public health problem among the Hispanic population.
Selected publications related to this project:
- Jiang H, Shin DH, Nguyen TT, Fueyo J, Fan X, Henry V, Carrillo CC, Yi Y, Alonso MM, Collier TL, Yuan Y, Lang FF, Gomez-Manzano C. Localized Treatment with Oncolytic Adenovirus Delta-24-RGDOX Induces Systemic Immunity against Disseminated Subcutaneous and Intracranial Melanomas. Clin Cancer Res 2019. 25(22):6801-6814. PMID: 31455679 PMCID: PMC6858961
- Martínez-Vélez N, Garcia-Moure M, Marigil M, González-Huarriz M, Puigdelloses M, Gallego Pérez-Larraya J, Zalacaín M, Marrodán L, Varela-Guruceaga M, Laspidea V, Aristu JJ, Ramos LI, Tejada-Solís S, Díez-Valle R, Jones C, Mackay A, Martínez-Climent JA, García-Barchino MJ, Raabe E, Monje M, Becher OJ, Junier MP, El-Habr EA, Chneiweiss H, Aldave G, Jiang H, Fueyo J, Patiño-García A, Gomez-Manzano C*, Alonso MM* [*, co-corresponding authors]. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat Commun 2019. 10(1):2235. PMID: 31138805 PMCID: PMC6538754
- Martinez-Velez N, Marigil M, García-Moure M, Gonzalez-Huarriz M, Aristu JJ, Ramos-García LI, Tejada S, Díez-Valle R, Patiño-García A, Becher OJ, Gomez-Manzano C, Fueyo J, Alonso MM. Delta-24-RGD combined with radiotherapy exerts a potent antitumor effect in diffuse intrinsic pontine glioma and pediatric high grade glioma models. Acta Neuropathol Commun 2019. 7(1):64. PMID: 31036068 PMCID: PMC6487528.
- Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, Gumin J, Vence LM, Wistuba I, Rodriguez-Canales J, Villalobos PA, Dirven CMF, Tejada S, Valle RD, Alonso MM, Ewald B, Peterkin JJ, Tufaro F, Fueyo J. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol 2018. 36(14):JCO2017758219. PMID: 29432077 PMCID: PMC6075856.
- Jiang H, Clise-Dwyer K, Ruisaard KE, Fan X, Tian W, Gumin J, Lamfers ML, Kleijn A, Lang FF, Yung WK, Vence LM, Gomez-Manzano C, Fueyo J. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS One 9(5):e97407, 2014. e-Pub 2014. PMID: 24827739 PMCID: PMC4020829.